t(11;14) is currently classified as a standard-risk genetic marker for newly diagnosed multiple myeloma (NDMM) patients. But some recent studies have suggested that t(11;14) may be an intermediate-risk marker in multiple myleoma (MM). The clinical significance of t(11;14) in MM is still controversial. Here, using high-resolution single nucleotide polymorphism-based mapping array (SNP-array) analysis and fluorescence in situ hybridization (FISH), the most common gained region of chromosome 11 was identified in 96/611 (15.7%) NDMM patients which located at 11q13.3-q25 (65.7Mb in size and contains 622 genes including CCND1). It demonstrated a statistically significant coexisting with t (11;14) (p<0.001), complex karyotypes (involves at least 6 chromosomes) (p<0.001), high plasma cell ratio in bone marrow (p=0.001) and poor response to VRD (bortezomib, lenalidomide, dexamethasone) treatment (p<0.001), but mutually exclusive with monosome 13, del(13q14), dup1q21 and t (4;14). When exploring the correlation of t(11;14) with genomic copy number variations (CNAs), it was also indicated that t(11;14) was most closely related to 11q gain (39%), while negatively correlated with others including gain of 1q and chromosome 3, 5, 6, 7, 9, 11, 15, 19, loss of 1p, 12p, 14q and chromosome 13 and 22.
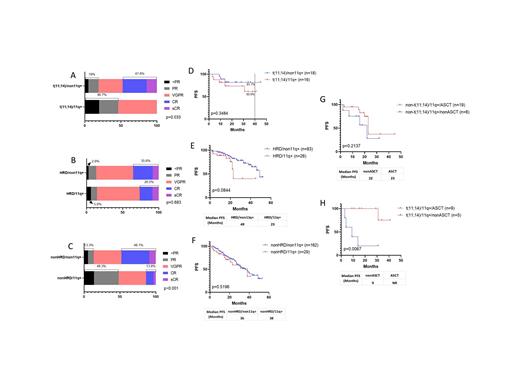
With a median follow up time of 22 months, survival analysis of 40 t(11;14) patients, 123 hyperdiploid (HRD) patients showed that 11q gain could have an adverse impact on the progression-free survival (PFS) in both groups. In t(11;14) subgroup, the median PFS (mPFS) were not reached, but PFS at 40 months were 60.9% vs 81.7% with 11q gain vs non-11q gain patients. In HRD subgroup, the mPFS were 23 months vs 49 months with 11q gain vs non-11q gain groups. Autologous hematopoietic stem cell transplantation (ASCT) can significantly improve the PFS of patients with t(11;14) and 11q gain (mPFS for transplant-eligible (TE) vs transplant-ineligible (TIE) patients were not reached vs 9 months, P=0.0067), but not helpful to 11q gain patients without t(11;14) (mPFS for TE vs TIE: 23 months vs 22 months, p=0.2137). Multivariate analysis confirmed that 11q gain was an independent adverse factor to PFS of MM with t(11;14) (Hazard ratio: 11.621, 95% CI: 1.027-131.507; p=0.048; n=37).
Conclusions: Gain of 11q13.3-q25 is a reproducible chromosomal copy number abnormality in NDMM characterized by coexisting with t (11;14), complex karyotypes, higher plasma cell ratio in bone marrow and poor treatment response to VRD. t(11;14) patients with 11q gain had a poorer PFS compared to patients with t(11;14) only, mPFS was only 9 months for TIE patients. t(11;14) with 11q gain NDMM patients needs to be considered as high risk and ASCT could improve patients' survival.
Disclosures
No relevant conflicts of interest to declare.